
In the recommended sets, the pH of the buffer solutions can be calculated eitherīy the Hückel or Pitzer method, and the pH predictions of these methods agree in most cases within 0.005 at least up to ionic

Several sets of phosphate buffer solutionsĪre recommended,e.g., for calibrations of glass electrode cells. In one of these methods, equations of the Hückel type are usedįor ionic activity coefficients and in the other equations of the Pitzer type. Ionic strength of about 0.5 mol-kg-1 can be accurately predicted by the two methods recommended. It is shown that all data used in the tests up to an Published thermodynamic data measured in aqueous mixtures of sodium or potassium dihydrogen phosphate with hydrogen phosphateĪnd chloride at 25☌ were used to test recently developed methods for calculation of the pH of phosphate buffer solutions.Įquations for ionic activity coefficients are used in these methods.
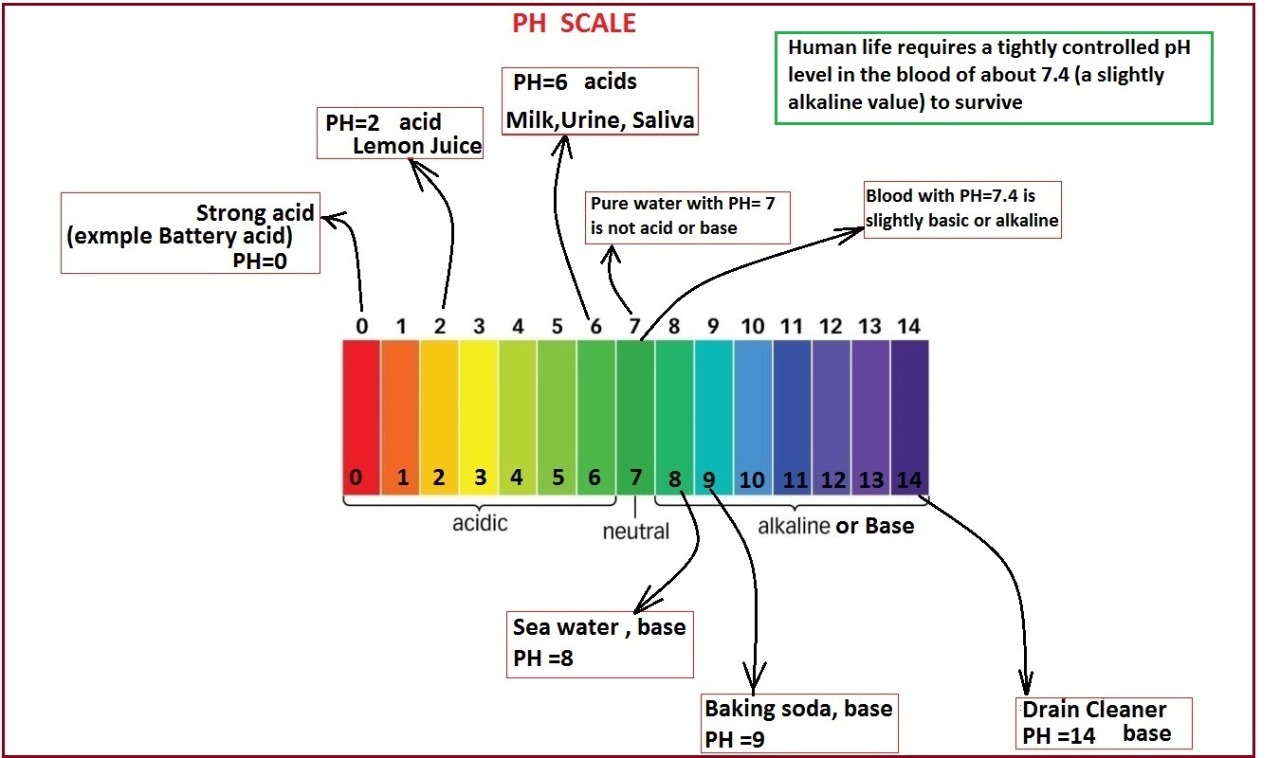
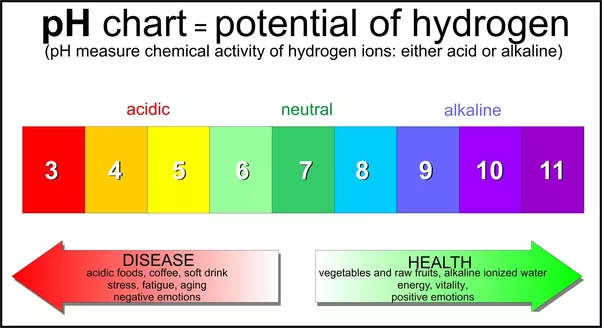
#PH WHEN YOU MIX BASE WITH WATER FULL#
Approximations are most accurate in waters of high total carbonate carbon and low ammonia concentrations, where the fourth-order approximation yields results that are within 0.05 pH units for the full range of pH values tested (5–10). the equations have been tested for salinities ranging from 0 to 35‰ (fresh to sea water), for temperatures ranging from 0 to 35☌, for total carbonate carbon concentrations of 0♱ and 5♰ mmol/liter, and for total ammonia nitrogen concentrations of 0 and 10 mg/liter. Three simplifications are tested based on a second-, a third-and a fourth-order polynomial equation for hydrogen ion concentrations. Because of the need for simplification of the equations to yield explicitly solvable polynomial equations, the accuracy of the solutions depends on the simplification made and varies with water properties.
#PH WHEN YOU MIX BASE WITH WATER TRIAL#
The method avoids trial and error estimations and results in a direct calculation procedure that can be implemented in models developed in various modeling environments, such as spreadsheets, conventional programming languages (BASIC, C, FORTRAN, PASCAL, etc.) or specialized modeling languages (Extend™, Stella™).The method developed is based on the solution of the full alkalinity-pH equation. The method is based on a fourth-order polynomial relationship between hydrogen ion concentration and other (conservative) water quality parameters. Due to its biodegradability and excellent sensor performance, the sensor is expected to find potential applications in human health monitoring, human motion detection, disease diagnosis and artificial intelligence.Ī procedure for the calculation of pH in fresh and salt waters has been developed. Under natural conditions, it can be completely dissolved in urban tap water containing 0.02% acetic acid and snail enzyme after 5 days, showing good natural degradability. The obtained degradable sensor exhibits excellent sensing performance, with a sensitivity of 1.7 kPa⁻¹, fast response time (180 ms), and good repeatability. This paper proposes a low-cost method to produce a high-performance biodegradable flexible capacitive sensor which composed of a degradable glycerol/chitosan dielectric layer with micro-protrusions and two degradable copper nanowire/chitosan electrodes. In recent years, the rapid development of wearable disposable electronic devices and the increasing awareness of environmental sustainability have put forward higher and higher new requirements for the natural degradability of current electronic systems. Generally, if the HCO3- concentration is higher in one solution and the Ca2+ concentration is higher in the other, mixing precipitation of CaCO3 will occur when they are combined. Mixing precipitation is controlled by simultaneous chemical equilibria.
Mixing precipitation in a broad sense refers to the mixing effect that reduces the solubility of CaCO3 in mixed water. Mixing precipitation in a strict sense refers to the mixing effect that makes the state of CaCO3 change from dissolution into precipitation, or the increases in the precipitability of CaCO3 in mixed water. Mixing precipitation can be classified into two major different types: mixing precipitation in a strict sense and mixing precipitation in a broad sense. Results show that mixing precipitation of CaCO3 can be produced by mixing between two unsaturated water samples with respect to CaCO3, two oversaturated water samples, an unsaturated water sample and an oversaturated water sample. The mixing precipitation of CaCO3 in natural waters was theoretically studied by the methods of chemical equilibrium calculation.


 0 kommentar(er)
0 kommentar(er)
